Oxidation Number of K
Place Markers let you know where in the sequence a particular random number falls by marking it with a small number immediately to the left. The donation of an electron is then 1.

Group 15 Elements Oxidation State And Trends In Chemical Properties
5 3 42 18 20 This is the default layout Research Randomizer uses.

. Write the oxidation number of each atom in the skeleton equation. An electrochemical CH oxidation strategy that exhibits broad substrate scope operational simplicity and high chemoselectivity is described. Redox reductionoxidation ˈ r ɛ d ɒ k s RED-oks ˈ r iː d ɒ k s REE-doks is a type of chemical reaction in which the oxidation states of substrate change.
Equate the increase and decrease in oxidation number on the reactant side. Get the oxidation number calculator available online for free only at BYJUS. Identify the atoms which undergo change in oxidation number.
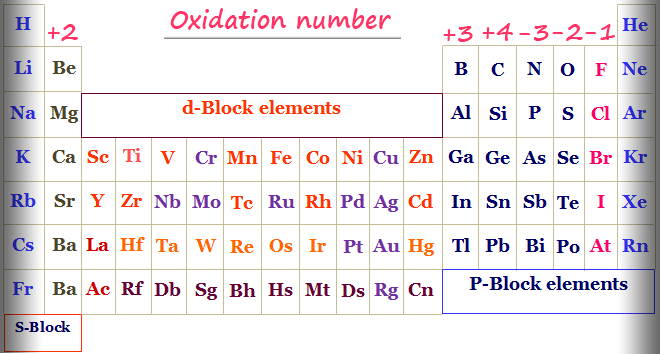
The alkali metals group I always have an oxidation number of 1. An atom that accepts an electron to achieve a more stable configuration is assigned an oxidation number of -1. Oxidation is the loss of electrons or an increase in the oxidation state of a chemical or atoms within it.
The oxidation number of hydrogen in a compound is 1 except in metal hydrides such as NaH when it is -1. Chlorine bromine and iodine usually have an oxidation number of 1 unless theyre in combination with oxygen or fluorine. While fully ionic bonds are not found in nature many bonds exhibit strong.
Learn how to use the oxidation number calculator with a step-by-step procedure. Reduction is the gain of electrons or a decrease in the oxidation state of a chemical or atoms within it. Reduction occurs when the oxidation number of an atom becomes smaller.
Oxidation occurs when the oxidation number of an atom becomes larger. Oxidation and reduction are therefore best defined as follows. The oxidation number of oxygen in a compound is -2 except in peroxides when it is -1.
2 17 23 42 50 Set 2. In chemistry the oxidation state or oxidation number is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionicIt describes the degree of oxidation loss of electrons of an atom in a chemical compoundConceptually the oxidation state may be positive negative or zero. The oxidation state of carbon increases from 2 to 4 while the oxidation state of the hydrogen decreases from 1 to 0.
The oxidation number of fluorine is always 1. Calculate the increase and decrease in oxidation number wrt reactant atoms. In fact our single-atom Pt catalyst was the most active for CO oxidation and PROX reactions among a number of supported Pt catalysts Supplementary Table S2.
It uses inexpensive and readily available materials. With Place Markers Off your results will look something like this. For example sodiums charge is 1 so its oxidation number is 1.
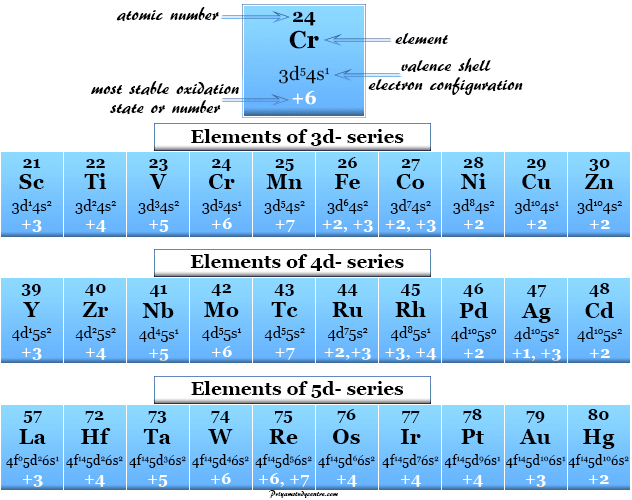
Keeping the atomic orbitals when assigning oxidation numbers in mind helps in recognizing that transition metals pose a special case but not an exception to this convenient method. The oxidation number of an atom in elemental form is 0. The oxidation number of a Group 1 element in a compound is 1.

Oxidation Number State Definition Rules How To Find And Examples

0 Response to "Oxidation Number of K"
Post a Comment